Cervical Fusion
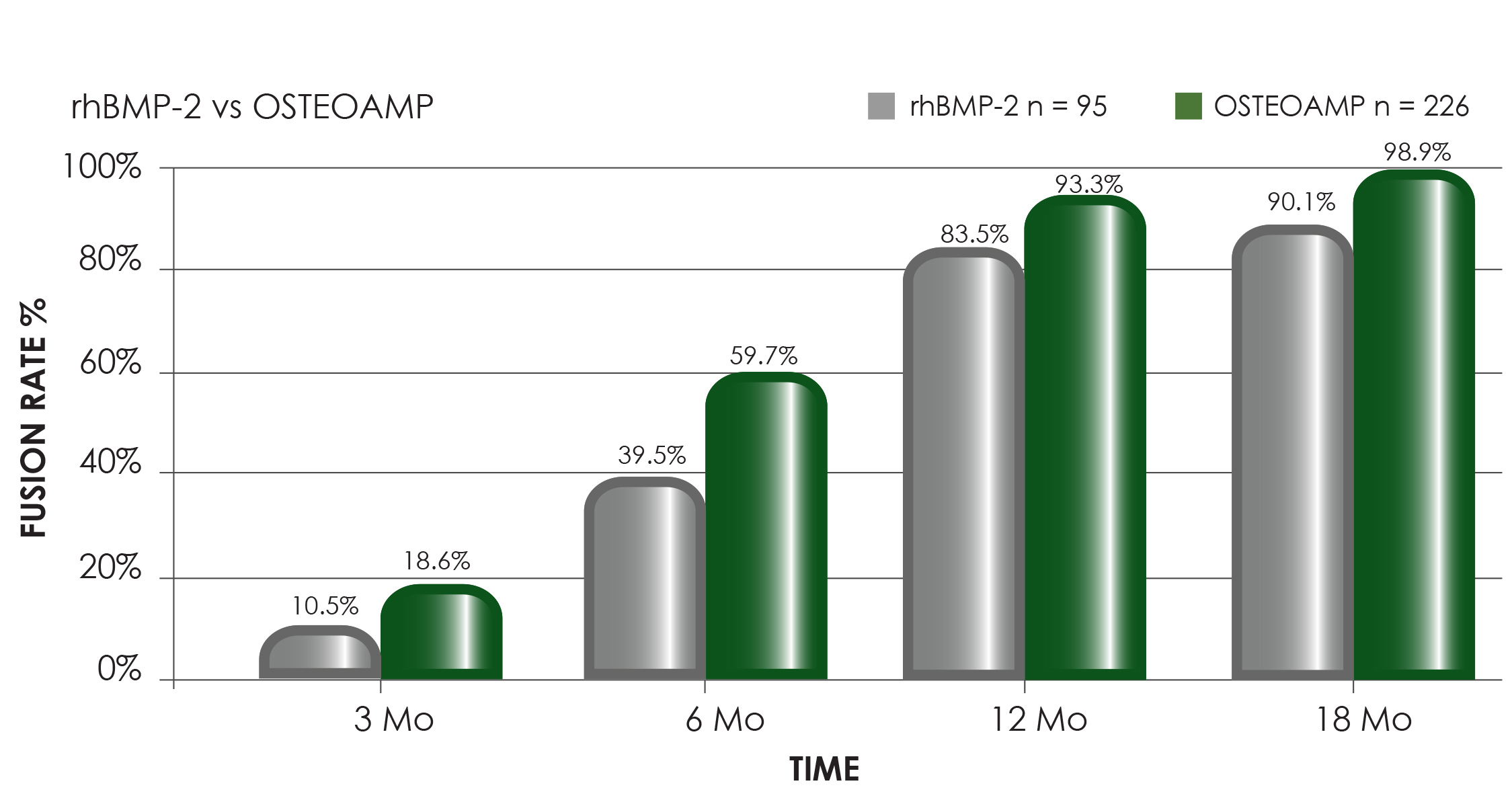
Radiographically determined fusion rates of 97.6% at 12 months and 100% at 18 months when OSTEOAMP was used, exceed fusion rates reported in the literature.3

OSTEOAMP is a differentiated allograft with unique processing designed to retain a wide array of growth factors* that support each stage of the bone healing cascade.1
*In vitro performance may not be predictive of performance in humans.
In vitro studies* show that OSTEOAMP retains up to 23 growth factors, including those present at each phase of the natural bone healing process.1
*In vitro performance may not be predictive of performance in humans.
All formats of OSTEOAMP undergo the unique and proprietary process and provide osteoconductive support along with osteoinductivity.

OSTEOAMP granules are mineralized corticocancellous allograft chips and exhibit osteoconductive and osteoinductive properties.
For optimal handling of the putty, bone marrow aspirate or blood are recommended. All formats may be rehydrated with bone marrow aspirate, blood, or saline.
*In vitro performance may not be predictive of performance in humans.
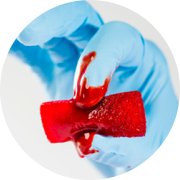
The OSTEOAMP Sponge is a malleable implant that readily conforms to various interbody devices and/or bony defects. An easy-to-use compressible sponge that combines both osteoconductive and osteoinductive properties.

To request more information on OSTEOAMP or to contact your local Bioventus Surgical representative to order, please fill out this form.
Instructions For Use
OSTEOAMP – DCI Doner Services Tissue Bank
OSTEOAMP – Lifelink Tissue Bank
OSTEOAMP – CTS
Material Management Packet
OSTEOAMP – Material Management Packet