Cell Migration
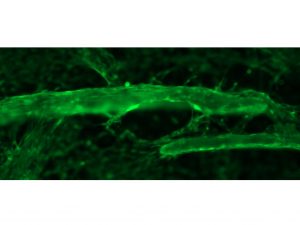
In a benchtop study over 5 days, seeded cells (highlighted in green) at 10X magnification demonstrate the ability to attach and migrate across OSTEOAMP SELECT Fibers.

OSTEOAMP is a differentiated allograft with unique processing designed to retain a wide array of growth factors* that support each stage of the bone healing cascade.1*
OSTEOAMP SELECT formats include flowable, fibers and sponges.
*In vitro performance may not be predictive of performance in humans.
*In vitro performance may not be predictive of performance in humans.

Versatile handling of OSTEOAMP SELECT Flowable satisfies the overwhelming need for a flowable biologic that stays where you put it, making it a great option for MIS procedures, thorough filling of the disc space, and grafting expandable and 3D-printed cages with ease.
*In vitro performance may not be predictive of performance in humans.
In a benchtop study over 5 days, seeded cells (highlighted in green) at 10X magnification demonstrate the ability to attach and migrate across OSTEOAMP SELECT Fibers.

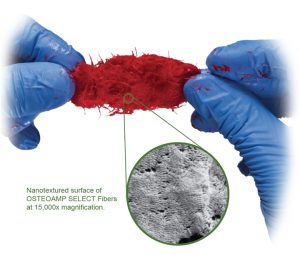
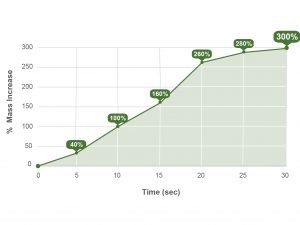
In a recent one-to-one fluid uptake test, OSTEOAMP SELECT Fibers rapidly hydrated.23
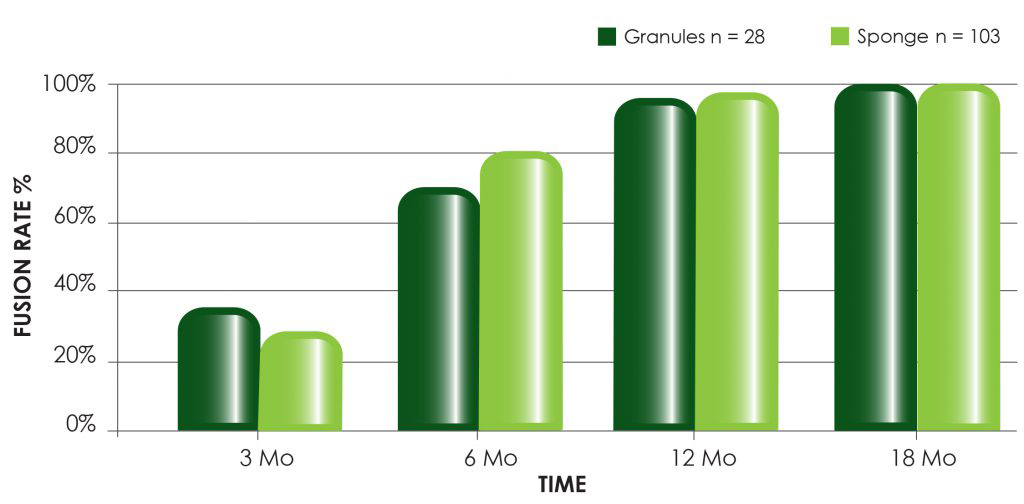
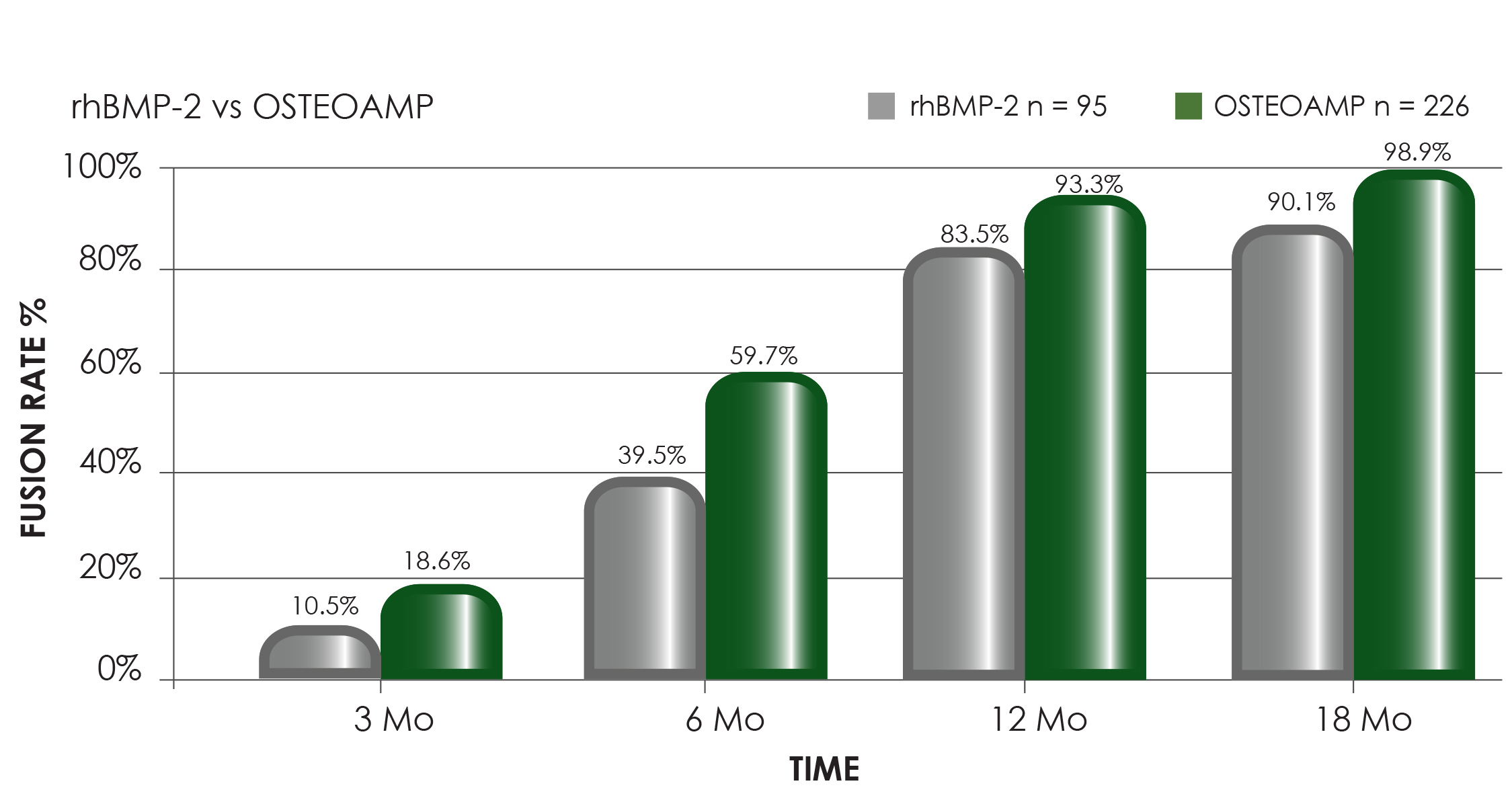
To request more information on OSTEOAMP or to contact your local Bioventus Surgical representative to order, please fill out this form.
Instructions For Use
OSTEOAMP – DCI Doner Services Tissue Bank
OSTEOAMP – Lifelink Tissue Bank
OSTEOAMP – CTS
Material Management Packet
OSTEOAMP – Material Management Packet